Synthesis of colloidal silica particles using Stöber process.
Overview of the synthesis mechanism
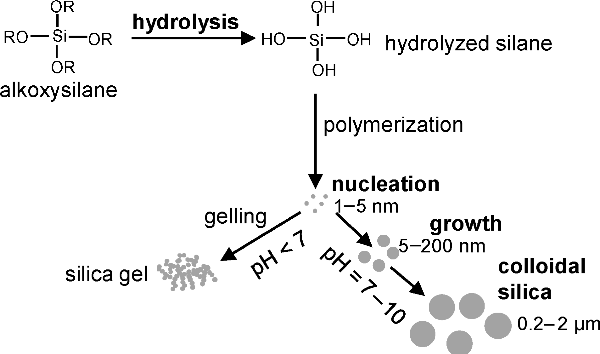 Mechanism of silica particle synthesis (Ref. 2)
Mechanism of silica particle synthesis (Ref. 2)
The colloidal SiO2 particles are generally synthesized using the sol-gel process. The process involves hydrolysis, followed by polycondensation (see the figure above). The liquid ammonia is used as a morphological catalyst. The particle sizes ranging from less than 5 nm to few micrometers can be synthesized by this process. The size of the final SiO2 strongly depends on type of solvent, catalyst, temperature, reaction time, rate of addition of reactant mixture (Ref. 1).
Chemicals:
- Tetraethy orthosilicate (TEOS)
- Ammonia solution (NH4OH)
- Ethanol
- DI Water
Synthesizing 420nm colloidal silica particles (modified from Ref. 5)
| Chemical | Volume |
|---|---|
| TEOS | 0.05 mL |
| Ethanol | 0.2 mL |
| Ammonia | 1 mL |
| Ethanol | 5 mL |
- Prepare a TEOS mixture by mixing TEOS into ethanol for the mentioned proportion (Table).
- Prepare an ammonia mixture by mixing ammonia into the ethanol (Table).
- Start stirring the ammonia and ethanol mixture using magnetic stirring at ambient temperature (Stirring speed at 500 rpm).
- Add TEOS and ethanol mixture quickly into ammonia solution under stirring.
- Close the glass vial with cap as tight as possible.
- Continue stirring for 2 hours.
- Immediately after the requisite reaction time is completed, wash the formed silica sol by repeated centrifugation (at 12000 rpm for 30 sec).
- After the solution attains a neutral pH, disperse silica particles into either ethanol or water.
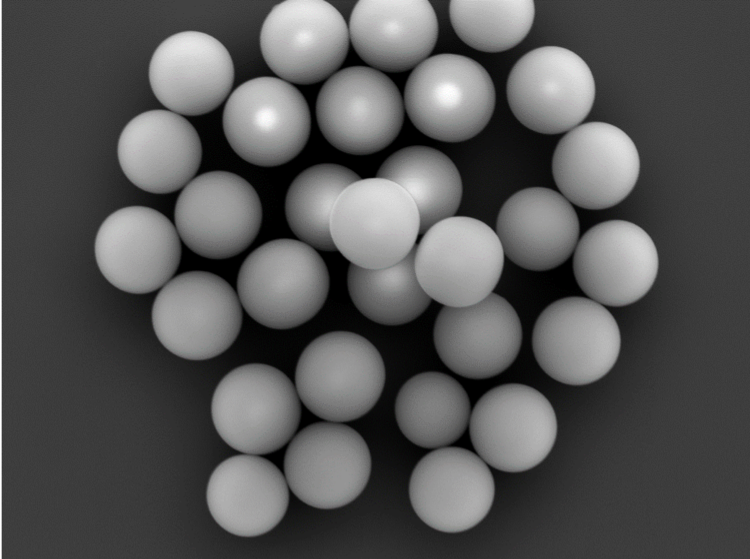 FESEM images of 420nm silica particles made through Stöber like process.
FESEM images of 420nm silica particles made through Stöber like process.
References:
- Mohd Qasim, et. al, Adv. Sci. Eng. Med. 2014, 6, 965-973.
- Frances Neville, et. al, J. Chem. Educ. 2012, 89, 940-942.
- Ismail Ab Rahman, et. al, J. of Nanomat. 2012, 1-15.
- Burcu Altin, Master of Science Thesis, 2009.
- J.H Zang, et. al, J. Mater. Res., 2003, 649-653.